The following Transcranial Magnetic Stimulation (TMS) systems have been cleared by the FDA to treat Major Depression and for additional indications.
Transcranial Magnetic Stimulation therapy systems (also called rTMS) are distinguished by 1) usability features that streamline the therapy experience for practitioners and patients; 2) the availability of different therapy waveforms which are associated with treatment times; 3) the availability of a neuro-navigated option for patients with MRIs.
A TMS session could last anywhere from 3 minutes to 37 minutes. rTMS session takes about 19 minutes with newer TMS devices and 37 minutes with older TMS devices. Conventional Deep TMS session takes about 20 minutes. iTBS session takes about 3 minutes for regular theta-burst stimulation. iTBS session takes about 9 minutes for accelerated TMS protocol.
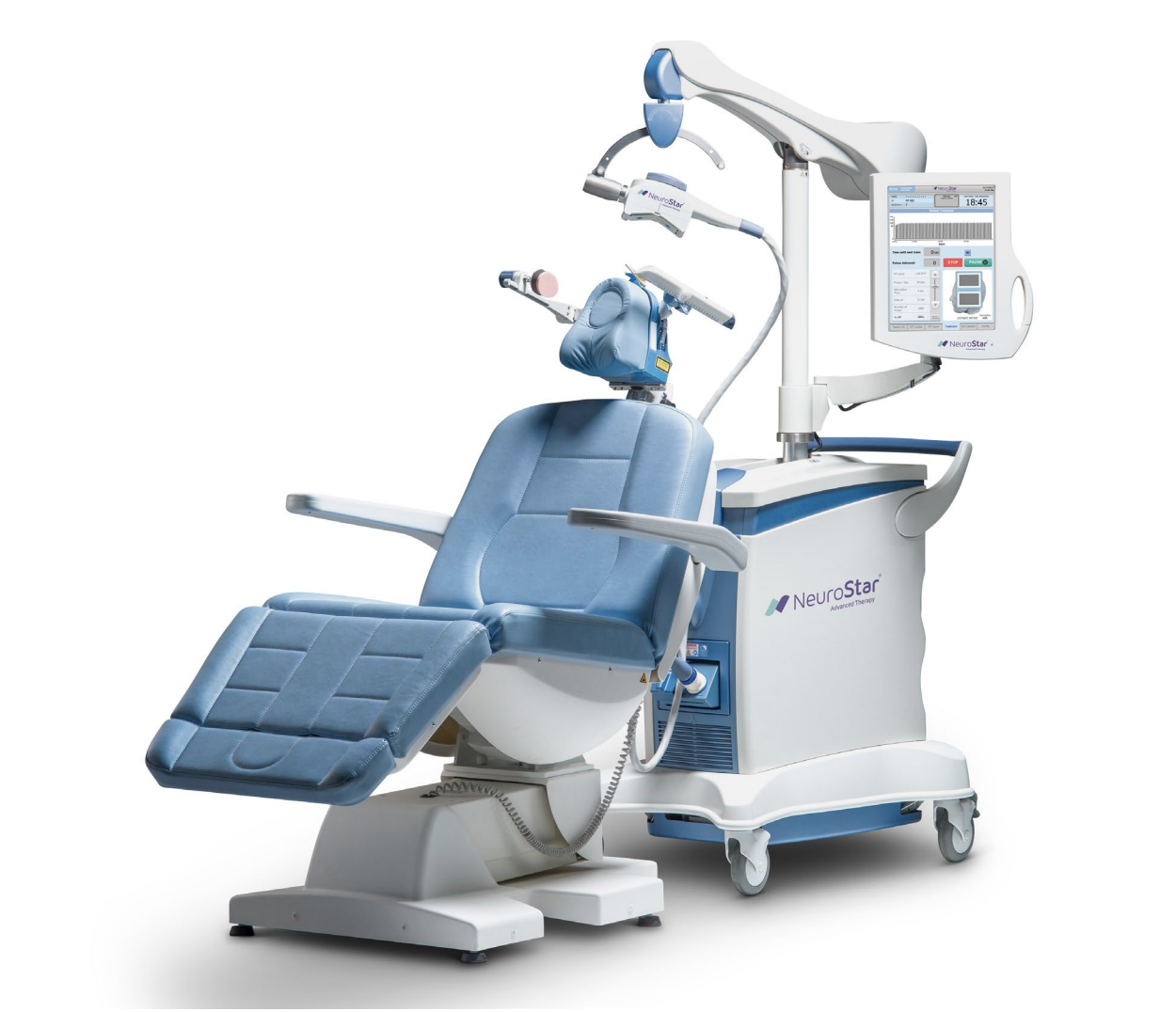
NeuroStar
Credit: neurostar.com
NeuroStar TMS is made by Neuronetics. Neuronetics sponsored the main pivotal trial published in Dec 2007 and got the FDA approval in 2008. In October 2008, the FDA approved TMS Therapy using the NeuroStar Advanced Therapy brain-stimulating device for patients suffering from Major Depressive Disorder who have failed to receive satisfactory improvement from antidepressant medication in the current episode. This make the NeuroStar rTMS system the first to be approved for depression treatment in the US which gave them “first mover” advantage in penetrating the US market. Neuronetics went on to pioneer clinical TMS practice in the US including a pivotal role in advocating for insurance coverage across the country. NeuroStar was also the first to receive FDA clearance for a 19-minute treatment time.
NeuroStar Advanced Therapy uses a figure-of- 8 coil. The system now has three treatment variations: standard, 37.5 minutes per session; DASH, 19 minutes per session, and TouchStar, which is an iTBS protocol.
NeuroStar system does not use a navigation system for MRI guided placement of the coil. NeuroStar has a special Contact Sensing strip that will alert the treater if contact between coil and head is not good. Neuronetics has a “pay-per-click” business model, which means that the TMS clinic has to effectively pay a fee for every session.
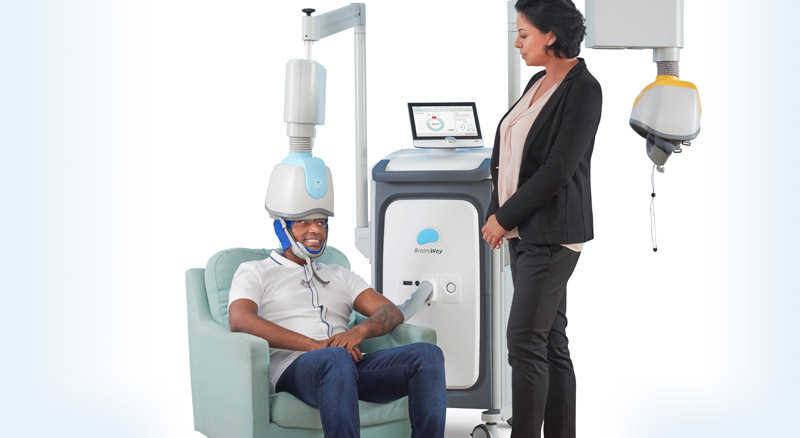
BrainsWay
Credit: brainsway.com
BrainsWay is based in Israel but does many of their clinical trials in the USA. BrainsWay TMS device does deep Transcranial Magnetic Stimulation (Deep TMS). Deep TMS uses a specially designed “H-shaped” coil which is housed inside a helmet. This design is intended to deliver stimulation deeper in the brain, hence deep TMS. Deep TMS coils do not work with any neuro-navigation systems. Brainsway FDA clearances and treatment are typically linked to specific coil designs.
The Brainsway H1 coil is FDA-cleared for Treatment Resistant Depression. The Brainsway H7-coil FDA-cleared for OCD. The Brainsway Deep Transcranial Magnetic Stimulation System H4 is FDA-cleared for helping short-term smoking cessation.
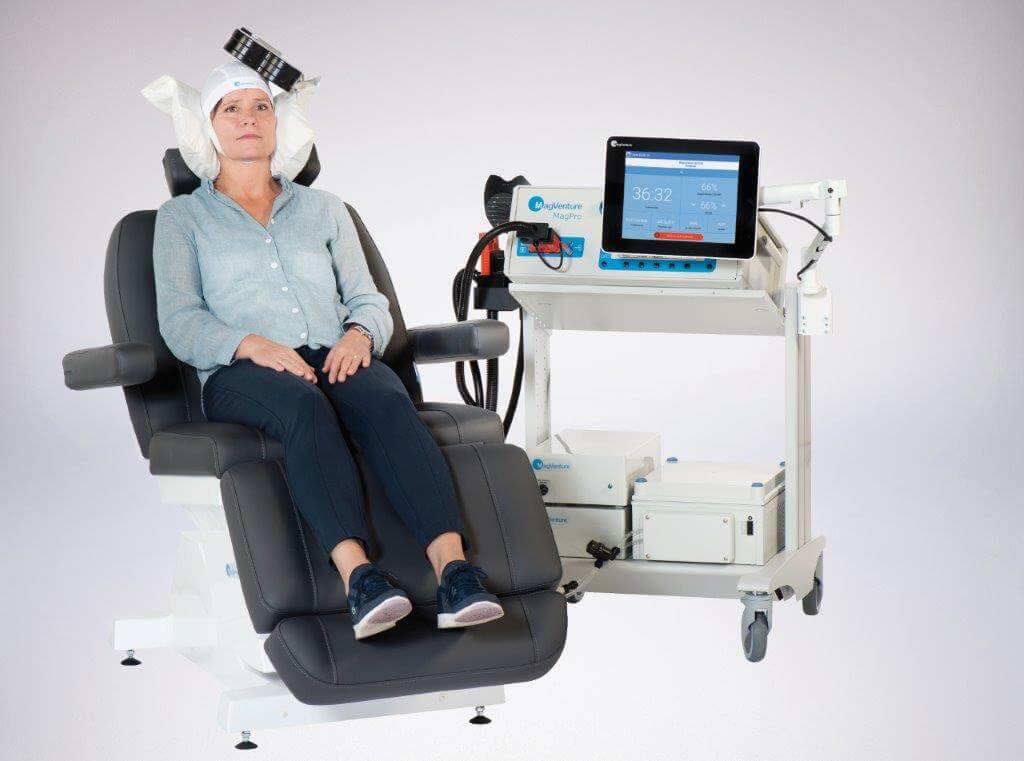
MagVenture
Credit: magventure.com
MagVenture is based in Denmark. They provide a range of systems to support the research market as well as clinical systems. MagVenture TMS Therapy was used among the largest clinical trials to with TMS to date (Blumberger et al, 2018, The Lancet: “THREE-D: a randomized non-inferiority trial”), comparing the standard, 37-minute TMS protocol to a newer, 3 minute Theta Burst protocol. The study concluded that the Theta Burst protocol is just as safe and effective for the treatment of major depressive disorder as standard TMS. In 2018, MagVenture received FDA clearance for the 3-minute protocol marketed as Express TMS. MagVenture is also FDA cleared for the standard 37-minute protocol as well as the 19-minute protocol. The MagVenture Cool D-B80 coil is FDA-cleared or adjunctive treatment of OCD.
Neurosoft (CloudTMS)

Credit: mycloudtms.com
Made by Russia based NeuroSoft, these rTMS systems are marketed as ClouldTMS in the US. The CloudTMS system has received FDA clearance for the 37.5 and 19-minutes treatment of Major Depressive Disorder. The CloudTMS based system is not neuro-navigated but can be using Soterix Medical system.
MAG & More Apollo TMS

Credit: magandmore.com
The Apollo TMS therapy system is developed and manufactured in Germany by MAG & More. The Apollo uses a marketed HANS positioning system and touch based Patient Management System. Apollo has received FDA clearance for the 37.5- and 19-minutes treatment of Major Depressive
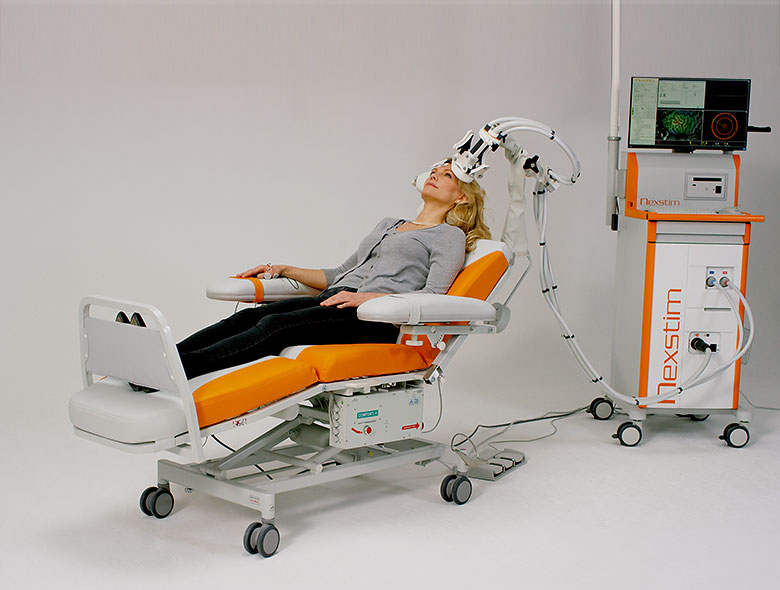
NexStim
Credit: nexstim.com
Nexstim is based in Finland. The “SmartFocus” system by Nexstim was originally developed to support neurosurgeons with brain mapping before performing brain surgery. A version of this system is FDA cleared for depression therapy. NexStim TMS is capable of rTMS, iTBS, and neuronavigation TMS which is marketed as NBT Navigated Brain Therapy.
MagStim
Credit: magstim.com
The medical engineers behind MagStim are some of the pioneers in the TMS industry. Magstim is based in the UK. Magstim is FDA cleared for depression treatment using the StimGuide system. Magstim also has FDA clearance for the iTBS 3-minute protocol. The Magstim Horizon rTMS system is FDA cleared for the 3-minute, 19-minute and 37.5-minute protocols and is indicated for the treatment of Major Depressive Disorder.
Soterix Medical
Credit: soterixmedical.com
Soterix Medical is based in the US. Soterix Medical bundles the Neurosoft TMS system with its own neuronavigation system that is unique for not requiring ‘line of site’. The system is capable of 37.5 and 19-minutes treatment and can be used with or without neuronavigation.
Brain Ultimate

Credit: brainultimate.com
Brain Ultimate offers a state-of-the-art TMS system developed in partnership with Shenzhen Yingchi Technology Co., Ltd., designed to deliver exceptional performance and clinical versatility. The system is FDA-cleared for the treatment of Major Depressive Disorder (MDD), Obsessive-Compulsive Disorder (OCD), and Intermittent Theta Burst Stimulation (iTBS) for MDD. Featuring a patented liquid-cooling circulation system, Brain Ultimate TMS ensures continuous operation throughout the day without the risk of overheating. The system supports both the standard 37-minute protocol and 19-minute protocol, and includes FDA-cleared OCD and accelerated iTBS treatment protocols for expanded clinical applications. Combining advanced technology, unlimited protocol flexibility, and a focus on clinician ease of use, Brain Ultimate TMS is built to elevate practices and support outstanding patient outcomes.
Magnus Medical
Credit: Magnus Medical
Magnus Neuromodulation System (MNS) with SAINT Technology developing a novel, rapid-acting brain stimulation technology designed to treat people suffering from major depressive disorder (MDD) who have not responded to existing treatments. The Magnus Medical “Model 1001k” applies the MagVenture rTMS and Brain Science Tools / Soterix Medical neuro-navigation.
Transcranial Magnetic Stimulation (TMS) Machines Comparison Table
| Magstim | Nexstim | Magventure | Neurostar | Brainsway | Apollo | CloudTMS | Soterix Medical | Magnus Medical | Brain Ultimate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard rTMS | ||||||||||
| iTBS | * | * | * | * | ||||||
| Neuro-Navigation | ||||||||||
| Depression | ||||||||||
| OCD | D-B80 | H7 | ||||||||
| Smoking | H4 | |||||||||
| Cost (Device, aggregated session) | $$ | $$$$ | $$ | $$$$ | $$$$ | $$ | $$ | $$$ | $$ |
● * The device is capable of iTBS but not FDA-cleared for iTBS ● Specific features will vary within each manufacturer by product. ● This table includes all possible features available. ● This table is a summary only, and the precise indications for use are limited by regulatory clearances.
The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
Subscribe to stay up to date about the latest in Neuromodulation and TMS